How to Enhance User Experience of Connected Devices for Digital Health Platforms
Feb 22, 2019
On January 15th, 2019 ClariMed led a workshop at the 11th Annual European Pre-Filled Syringes and Injectable Drug Devices conference by SMi, in London. At the conference, new technologies and innovations for platform systems of injectable delivery devices as well as the impact of digitalization and patient centricity with regulatory compliance were explored.
Clark’s workshop focused on connected devices and digital health as well as how to navigate the US FDA usability engineering requirements. Here’s an overview of what you missed!
Connected Devices and Digital Health - Current and Future Applications
Connected medical devices are medical devices that exchange and use information with at least one other product, technology, or system. There are several different types of connected medical devices such as diagnostic, monitoring, portable, therapeutic, surgical, and combination. An example of a connected device would be a glucose monitor that has a connected mobile phone application or an insulin pump. A glucose monitor on its own would not be considered a connected device because it is a stand-alone medical device.
There are many potential uses of connected devices for manufacturers, patients and caregivers, health care providers (HCPs) and caretakers, and large organizations and universities. Connected devices would allow for close patient monitoring as well as access to large anonymized data sets for research. Ultimately, connected devices have profound potential in future applications.
Overview of Applicable US FDA Usability Engineering Requirements & Regulatory Requirements for Connected Devices & Digital Health
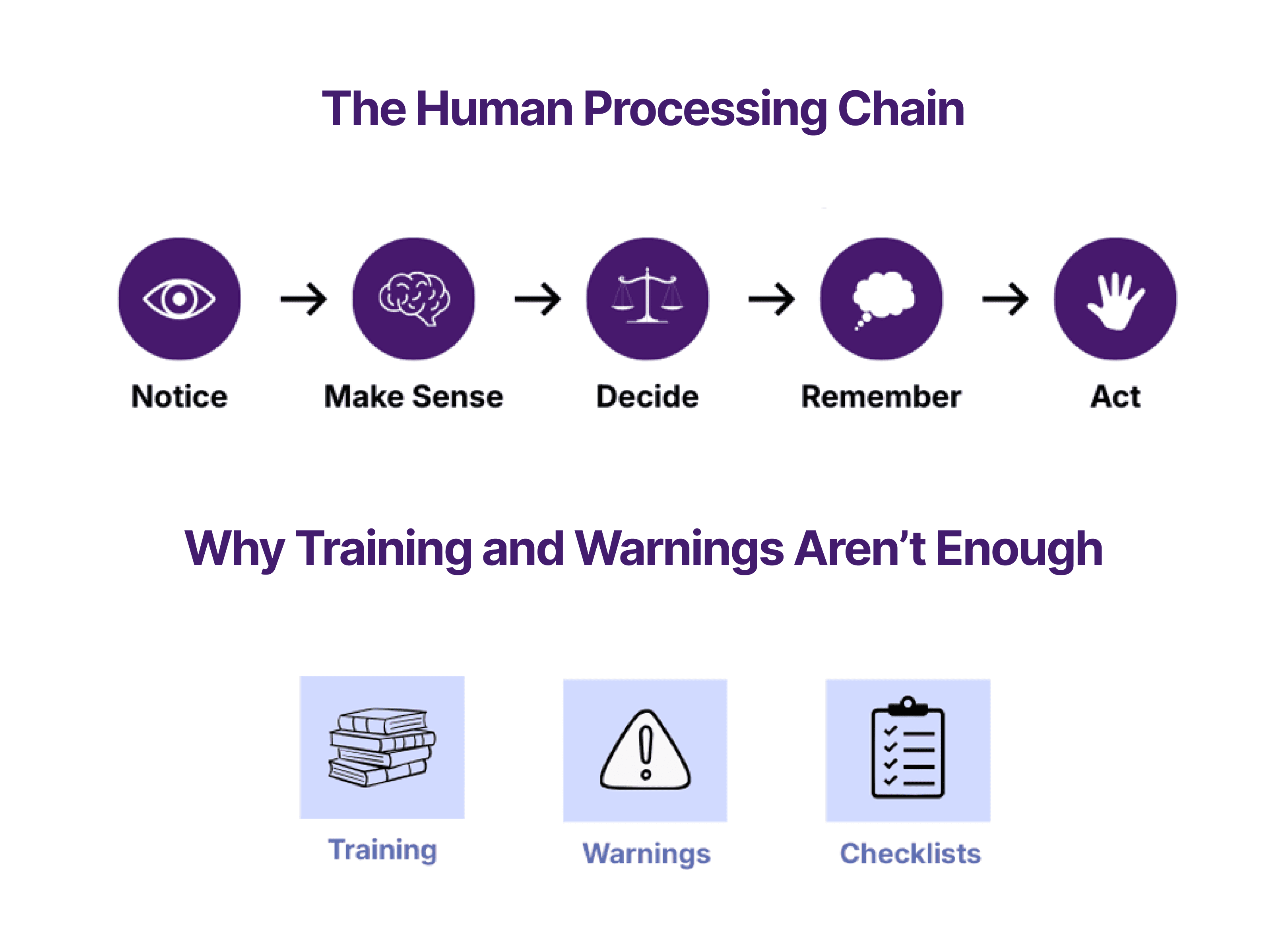
The FDA calls the ability to connect devices and systems “medical device interoperability,” and supplied a guidance document in 2017 with the purpose of providing design considerations as well as recommendations for information to include in pre-market submissions and device labeling.
As part of risk management, the FDA recommends focusing on potential hazards, safety concerns, and security issues associated with having an electronic interface. Safety must be maintained during normal and fault operations. It is suggested to mitigate failures or malfunctions caused by direct or indirect connection of intended devices, invalid commands, receiving and processing erroneous data or commands, and not adhering to the non-functional requirements of the communication specification.
Check out the slideshow which included case studies that highlight unique usability engineering considerations related to connected devices.
Whether you are developing a medical device that interfaces with a digital application or other devices, ClariMed stands ready to assist you in addressing usability engineering and human factors considerations that are unique to your connected medical device. Contact ClariMed today to learn how we can help.