
BLOG
Insights & Innovation
Explore our latest articles, case studies and expert perspectives on the medical device industry.

BLOG
Insights & Innovation
Explore our latest articles, case studies and expert perspectives on the medical device industry.

BLOG
Insights & Innovation
Explore our latest articles, case studies and expert perspectives on the medical device industry.

When Outliers Matter Most: Human Factors and User Safety
When Outliers Matter Most: Human Factors and User Safety
Feb 18, 2026
Human factors design and fundamental research share the same roots — but they differ in what they optimise for. This article explores why averages drive research while outliers drive safety, and why designing for the "typical" user can fail in the moments that matter most.
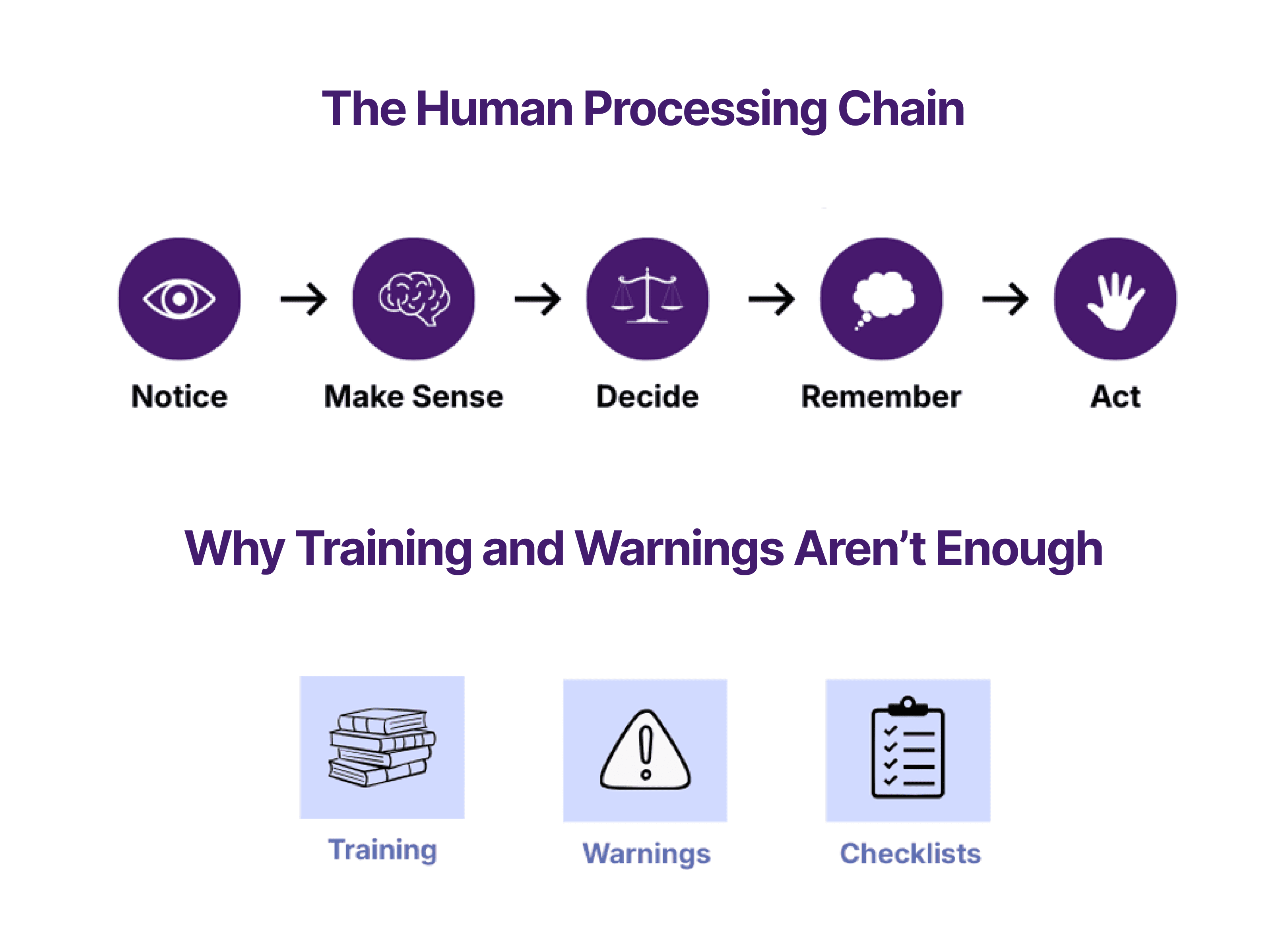
How humans actually use medical devices — and where usability breaks down
How humans actually use medical devices — and where usability breaks down
Jan 29, 2026
Use errors aren't random—they follow predictable patterns shaped by attention, memory, and motor limits. This post breaks down the five stages where medical device usability fails and why these issues keep surfacing in validation, regulatory review, and post-market use.

Lessons from the Caregiver's Perspective: Essential Usability Principles for Medical Device Design
Lessons from the Caregiver's Perspective: Essential Usability Principles for Medical Device Design
Jan 8, 2026
Through firsthand caregiving experience, this post reflects on the quiet moments and pressures of caring for a loved one, and how medical device usability can ease or intensify that burden. It highlights seven principles that support safer, more humane care at home.

ISO 13485 Year-End Requirements: Complete Compliance Checklist for 2026
ISO 13485 Year-End Requirements: Complete Compliance Checklist for 2026
Oct 15, 2025
Are your ISO 13485 annual requirements complete? This guide covers 6 critical compliance activities auditors verify during surveillance audits: internal audits, management reviews, supplier evaluations, and more. Plus how to avoid year-end scrambles.

How My Breast Cancer Journey Made Me a Better Human Factors Engineer
How My Breast Cancer Journey Made Me a Better Human Factors Engineer
Oct 2, 2025
A Human Factors Engineer reflects on her breast cancer journey and how it reshaped her empathy, focus on accessibility, and commitment to designing medical devices that truly support patients during their most difficult moments.

Communicating User Interface Issues: Why CCs and Ds Really Matter
Communicating User Interface Issues: Why CCs and Ds Really Matter
Sep 16, 2025
Use errors are only part of the story in medical device usability. Close calls (CCs) and use difficulties (Ds) highlight hidden risks that impact safety, design, and regulatory compliance. Learn why CCs and Ds matter just as much as errors in Human Factors evaluations.

When it comes to simulated use environments, how realistic is realistic enough?
When it comes to simulated use environments, how realistic is realistic enough?
Aug 6, 2025
Explore how realistic your simulated use environment needs to be for medical device usability testing. Learn when high-fidelity simulation is essential versus when lower fidelity suffices, plus key factors to consider for both formative and validation testing success.

Unlocking Innovation Through Early Human Factors Engagement
Unlocking Innovation Through Early Human Factors Engagement
Jul 18, 2025
Many promising medical devices fail due to poor user adoption, not technical issues. This article reveals why early human factors research is crucial, offering practical guidance on engaging HF experts to reduce costs and accelerate innovation.

Designing Medical Devices for People with Autoimmune Diseases: Embracing Flexibility and Empathy
Designing Medical Devices for People with Autoimmune Diseases: Embracing Flexibility and Empathy
Jul 10, 2025
When fatigue, pain, and brain fog strike without warning, medical devices must adapt. Learn how human factors engineering creates tools that support people with autoimmune conditions through their daily variability, honoring both their limitations and resilience.

The Role of UX/UI in Medical Device Safety and Adoption
The Role of UX/UI in Medical Device Safety and Adoption
Jul 3, 2025
When designing medical device UIs, closing the gap between what users intend to do & what actually happens is critical for safety. Learn the psychology behind user-device interaction and discover proven strategies for creating interfaces that healthcare professionals trust.

Location Location Location: Why Geography Matters in Human Factors Engineering
Location Location Location: Why Geography Matters in Human Factors Engineering
Jun 27, 2025
Medical devices designed for one market often fail in others. This article explores why geo differences in healthcare systems, user roles, and workflows can make or break your device's global success. Learn how to avoid costly mistakes with multi-location validation.

What If You Could See What I See? Using AI to Elevate Medical Device Design
What If You Could See What I See? Using AI to Elevate Medical Device Design
Hajni Salazar-Velekey shares her vision impairment experience and how AI could simulate these challenges for device design. The article examines fluctuating vision's impact on medical device use and proposes AI simulation to create more inclusive, user-centered products.

Maximising design consistency and existing mental models to take advantage of 'positive transfer'
Maximising design consistency and existing mental models to take advantage of 'positive transfer'
Jun 5, 2025
Discover how to leverage "positive transfer" in medical device UI design. Learn to build interfaces that feel intuitive by aligning with users' existing mental models, reducing errors and training time while improving patient safety through consistent design principles.

Navigating FDA Q-Sub Feedback on Human Factors Validation: A Guide to Moving Forward When Plans Don't Align
Navigating FDA Q-Sub Feedback on Human Factors Validation: A Guide to Moving Forward When Plans Don't Align
May 28, 2025
Learn how to respond when FDA questions your Human Factors Validation plan. This guide shows you how to clarify your approach, make the most of Q-Sub meetings, and use post-meeting discussions to align with reviewers and advance your medical device.

Human Factors Glossary for Medical Devices
Human Factors Glossary for Medical Devices
May 20, 2025
Navigate the complex world of human factors engineering with confidence. This comprehensive glossary defines essential terminology for medical device development, from basic concepts like usability to advanced tools like URRA. Perfect for newcomers to the field.

Understanding IRB Requirements for Usability Studies Involving Medical Devices
Understanding IRB Requirements for Usability Studies Involving Medical Devices
May 12, 2025
Navigate IRB requirements for medical device usability studies. Learn when FDA approval is needed, understand human subjects research criteria, and get best practices for compliance.

Why Cybersecurity Is Non-Negotiable for Medical Devices
Why Cybersecurity Is Non-Negotiable for Medical Devices
Apr 22, 2025
Explore how ClariMed helps medtech companies strengthen cybersecurity for connected medical devices. From secure design to compliance, we support innovation with confidence.

The Importance of Human Factors Engineering in Post-Market Surveillance
The Importance of Human Factors Engineering in Post-Market Surveillance
Apr 22, 2025
Discover how Human Factors Engineering strengthens post-market surveillance by analyzing user interactions, identifying safety risks, and improving device design. Learn practical methods to incorporate HFE for better outcomes and reduced recalls.

UOUP Decoded: When to Leverage Legacy Interfaces in Medical Device Development
UOUP Decoded: When to Leverage Legacy Interfaces in Medical Device Development
Apr 3, 2025
Explore the User Interface of Unknown Provenance (UOUP) framework for medical devices. This article unpacks when to leverage legacy interfaces, the benefits and limitations, and how manufacturers can navigate evolving regulatory expectations in a post-IEC 62366-1:2015 world.

The Perils of Medical Device Predicates and User Interface Design
The Perils of Medical Device Predicates and User Interface Design
Mar 18, 2025
Medical devices rely on predicate devices for FDA approval, but this can impede user interface innovation. Learn how the 510(k) pathway affects patient safety, usability, and healthcare costs—and how prioritizing modern UI design principles can create safer devices.

Demystifying the FDA Q-Sub Process for Human Factors Deliverables
Demystifying the FDA Q-Sub Process for Human Factors Deliverables
Mar 11, 2025
Discover how to navigate the FDA Q-Submission process for Human Factors Engineering. This guide outlines key steps, strategic approaches during regulatory uncertainty, and ways to maximize FDA feedback before formal medical device submission.

The Invisible Half: Uncovering & Addressing Gender Data Bias in Medical Devices
The Invisible Half: Uncovering & Addressing Gender Data Bias in Medical Devices
Mar 8, 2025
Medical devices often overlook gender differences in design, putting women at risk. This article explores gender data bias in medical technology, regulatory gaps, and solutions for creating inclusive devices. Part of our "Designing for All" series on healthcare inequality.

A Global perspective: Maximizing Cultural Inclusivity
A Global perspective: Maximizing Cultural Inclusivity
Feb 26, 2025
Designing medical devices with cultural sensitivity creates better outcomes worldwide. This article explores why inclusivity matters, key cultural factors to consider, and best practices for implementing cultural diversity in your design process.

Bespoke Software Development: Partnering with Experts to Drive Healthcare Innovation
Bespoke Software Development: Partnering with Experts to Drive Healthcare Innovation
Jan 21, 2025
Healthcare organizations face complex software development challenges across multiple pipelines. This article explores how strategic partnerships help manage legacy systems, drive innovation, and maintain efficiency - without overburdening internal teams.


Cybersecurity in Healthcare: Building Resilient Medical Devices
Cybersecurity in Healthcare: Building Resilient Medical Devices
Jan 8, 2025
Expert digital health services for medical device and pharma companies. From UI/UX design to software development, cybersecurity, and validation. Bringing innovation to market safely.

FDA's New Guidance is a Game-Changer for AI Medical Devices
FDA's New Guidance is a Game-Changer for AI Medical Devices
Dec 18, 2024
The FDA's new PCCP guidance revolutionizes how AI medical devices can evolve after market approval. Discover how this framework enables continuous improvement while maintaining patient safety standards.

Getting to the Heart of Medical Device Design: Why Understanding Lived Experiences Matters
Getting to the Heart of Medical Device Design: Why Understanding Lived Experiences Matters
Dec 4, 2024
Learn how medical device design is shaped by patient lived experiences through specialized research environments and methods, ensuring solutions meet both functional needs and emotional comfort.

The Power of User-Friendly Negative Pressure Wound Therapy (NPWT): Enhancing Patient Compliance
The Power of User-Friendly Negative Pressure Wound Therapy (NPWT): Enhancing Patient Compliance
Nov 19, 2024
Discover how user-friendly NPWT device design boosts patient compliance and treatment success. Learn why intuitive interfaces matter in wound care & how smart design features enhance healing outcomes.

A Global Perspective: Why Diversity Matters in Human Factors Engineering (HFE)
A Global Perspective: Why Diversity Matters in Human Factors Engineering (HFE)
Nov 13, 2024
Learn how diverse human factors engineering strategies drive global product success. Discover practical steps to implement inclusive HFE practices and expand your market reach.


10 Critical Tips for Acing Your ISO 13485:2016 Certification Audit
10 Critical Tips for Acing Your ISO 13485:2016 Certification Audit
Aug 26, 2024
Master ISO 13485:2016 certification with ClariMed's expert tips. Enhance your medical device QMS for regulatory compliance and quality assurance.

5 Document Control Best Practices That Can Revolutionize Your Product Development
5 Document Control Best Practices That Can Revolutionize Your Product Development
Aug 15, 2024
Explore 5 document control best practices to enhance product development. Learn how to streamline operations, improve compliance, and revolutionize your quality management process.

Uncovering Use Errors via a Known Use Problem Summary (KUPS)
Uncovering Use Errors via a Known Use Problem Summary (KUPS)
Apr 10, 2023
Known Use Problem Summary: A crucial tool for identifying and mitigating medical device use-related risks.

Medical Devices vs. Combination Products
Medical Devices vs. Combination Products
Jan 31, 2023
Key differences in human factors processes for medical devices vs. combination products: FDA perspective.

2023 FDA Labeling Draft Guidence
2023 FDA Labeling Draft Guidence
Jan 24, 2023
FDA issues draft guidance on dosage labeling for prescription drugs and biologics; comments due 3/14/2023.

Formative Evaluations Series: Early Usability Evaluations to Optimize Medical Product Design
Formative Evaluations Series: Early Usability Evaluations to Optimize Medical Product Design
Jan 3, 2023
Optimize medical device usability: Formative evaluations guide design, reduce costs, and improve outcomes.

New NMPA Human Factors Guidelines for Medical Devices in China: What Manufacturers Need to Know
New NMPA Human Factors Guidelines for Medical Devices in China: What Manufacturers Need to Know
Jul 27, 2022
Comparing NMPA and FDA human factors guidance: Key differences in medical device regulation in China vs US.

Updated FDA Guidance: Drug and Biologic Instructions for Use
Updated FDA Guidance: Drug and Biologic Instructions for Use
Jul 14, 2022
FDA updates drug and biologic IFU guidance: Key changes in content, readability, and usability requirements.

Human Factors Return on Investment Through the Lens of Medical Device Recalls
Human Factors Return on Investment Through the Lens of Medical Device Recalls
Jun 15, 2022
Medical device recalls: Rising trends, financial impacts, and how human factors engineering can mitigate risks.

A Guide to FDA 483s and Warning Letters
A Guide to FDA 483s and Warning Letters
May 31, 2022
FDA inspections, Form 483s, and warning letters: Implications for medical device manufacturers and prevention.

Best Practices for Medical Device User Training
Best Practices for Medical Device User Training
Apr 21, 2021
Medical device user training: Key findings on effective methods for healthcare providers from ClariMed study.

FDA Training Decay Pilot Study - Results & Discussion
FDA Training Decay Pilot Study - Results & Discussion
Jan 21, 2021
FDA-funded study by ClariMed explores training decay in medical device usability. Pilot results show task success varies with training periods.

How Will Brexit Affect the Implementation of Human Factors Engineering for Your Device?
How Will Brexit Affect the Implementation of Human Factors Engineering for Your Device?
Dec 31, 2020
Examine Brexit's impact on medical device usability regulations. Discover key differences between UK and EU requirements for implant information and post-market surveillance under the new MDR.


Applying Human Factors Engineering to Combination Products
Applying Human Factors Engineering to Combination Products
Dec 27, 2020
Key human factors considerations for combination products: Comparing CDER and CDRH FDA guidance.


Mastering FDA Guidelines: Bridging Human Factors Data for Combination Products
Mastering FDA Guidelines: Bridging Human Factors Data for Combination Products
Dec 15, 2020
FDA guidance on bridging human factors data for combination products: Analysis and submission tips.

FDA Q&A Regarding COVID-19 In-Vitro Diagnostic Tests (May 13, 2020)
FDA Q&A Regarding COVID-19 In-Vitro Diagnostic Tests (May 13, 2020)
May 13, 2020
On May 13, 2020, the FDA hosted a Virtual Town Hall for COVID-19 test manufacturers, addressing updates to Emergency Use Authorization (EUA) guidelines and validation for antigen tests.

Improving Complaint Management for Medical Devices Under EUAs with Human Factors
Improving Complaint Management for Medical Devices Under EUAs with Human Factors
Apr 27, 2020
A guide to enhancing medical device complaint management through human factors integration. Improve data collection, analysis, and resolution of use-related issues.

FDA COVID-19 EUA Resources
FDA COVID-19 EUA Resources
Apr 14, 2020
A summary of FDA's latest COVID-19 updates, including EUA process, N95 mask reuse, and upcoming town halls on diagnostic tests. Essential info for medical device manufacturers.

FDA Guidance Relating to Clinical Usability Studies During the COVID-19 Pandemic
FDA Guidance Relating to Clinical Usability Studies During the COVID-19 Pandemic
Mar 19, 2020
A comprehensive overview of FDA's guidance for clinical trials and usability studies during COVID-19, covering safety measures, protocol changes, and reporting requirements.

FDA Enforcement Policy and Emergency Use Authorization (EUA) for Face Masks and Respirators
FDA Enforcement Policy and Emergency Use Authorization (EUA) for Face Masks and Respirators
Feb 4, 2020
A comprehensive overview of FDA's regulatory changes for face masks and respirators in response to COVID-19, including Enforcement Policy and Emergency Use Authorizations.

ISO 14971:2019—Which Changes Impact Human Factors?
ISO 14971:2019—Which Changes Impact Human Factors?
Dec 31, 2019
A comprehensive overview of ISO 14971:2019 updates, focusing on key changes in risk management processes and their impact on human factors engineering for medical devices.

Human Factors Validation Study Guidelines for Medical Devices
Human Factors Validation Study Guidelines for Medical Devices
Dec 23, 2019
A comprehensive overview of the 2016 FDA Human Factors Guidance, covering key requirements for medical device validation testing and submission.

Perceived Quality—What it is and Why it is Important
Perceived Quality—What it is and Why it is Important
Dec 23, 2019
An in-depth look at Human Factors Engineering for FDA submissions and the importance of perceived quality in medical device design, development, and user experience.

Reverse Human Factors Engineering
Reverse Human Factors Engineering
Oct 25, 2019
Discover how Reverse Human Factors Engineering enhances medical device clinical trials by minimizing use errors and tracking human variability for better outcome analysis.

Redefining "Normal Use" for Medical Devices
Redefining "Normal Use" for Medical Devices
Jul 29, 2019
ClariMed proposes a new definition for "Normal Use" in medical device usability, addressing real-world user behaviors and improving usability validation testing methods.


FDA’s Dr. Feng Presents on Do’s and Don’ts for Human Factors Data in CDRH Premarket Submissions
FDA’s Dr. Feng Presents on Do’s and Don’ts for Human Factors Data in CDRH Premarket Submissions
May 14, 2019
Expert advice from FDA's Human Factors Reviewer on improving medical device submissions. Covers risk analysis, validation testing, and addressing FDA feedback.

Medical Device Learnings from the April 2019 IEC Joint Working Group Meeting
Medical Device Learnings from the April 2019 IEC Joint Working Group Meeting
Apr 13, 2019
ClariMed shares key takeaways from the IEC 62366-1:2015 update, covering unintended users, lay vs. professional use, and symbol usage in medical device usability standards.

How to Enhance User Experience of Connected Devices for Digital Health Platforms
How to Enhance User Experience of Connected Devices for Digital Health Platforms
Feb 22, 2019
ClariMed's insights on connected medical devices, digital health applications, and FDA usability requirements from their workshop at the SMi conference in London.

Interview with Jeff Durney, Ambulatory Human Factors Specialist
Interview with Jeff Durney, Ambulatory Human Factors Specialist
Jun 7, 2018
Jeff Durney's perspective on applying aviation safety principles to healthcare, focusing on surgical checklists, training, and safety reporting to improve patient outcomes.

Cognitive Bias in Usability Testing
Cognitive Bias in Usability Testing
Dec 22, 2016
An overview of cognitive bias in usability studies, its potential impact on results, and strategies for identifying and mitigating these subconscious influences.

Cliff Notes: FDA Draft Guidance for Human Factors (2011)
Cliff Notes: FDA Draft Guidance for Human Factors (2011)
Aug 13, 2015
A detailed guide to the FDA's human factors engineering process for medical devices, covering user research, risk analysis, prototyping, testing, and regulatory submission.

The FDA’s 2011 Draft Guidance vs. 2000 Guidance for Human Factors
The FDA’s 2011 Draft Guidance vs. 2000 Guidance for Human Factors
Aug 13, 2015
An in-depth look at how FDA guidance on human factors in medical device design evolved from 2000 to 2011, highlighting key changes and their significant impact on manufacturers.

FDA 2011 Human Factors Draft Guidance vs. IEC 62366-1:2015
FDA 2011 Human Factors Draft Guidance vs. IEC 62366-1:2015
Aug 13, 2015
This post analyzes differences between IEC 62366-1:2015 and FDA's 2011 Human Factors Guidance for medical devices, covering unique aspects of each standard for usability engineering.

Usability Engineering for Medical Devices 101
Usability Engineering for Medical Devices 101
Apr 26, 2015
A comprehensive guide to the 5 key phases of Usability Engineering for medical devices, covering research, risk analysis, testing, validation, and regulatory compliance.
Let's work together!
Contact us to discuss how we can advance your medical device development efforts together.

Let's work together!
Contact us to discuss how we can advance your medical device development efforts together.

Let's work together!
Contact us to discuss how we can advance your medical device development efforts together.

ClariMed, Inc. 2026. All rights reserved.

ClariMed, Inc. 2026. All rights reserved.

ClariMed, Inc. 2026. All rights reserved.
